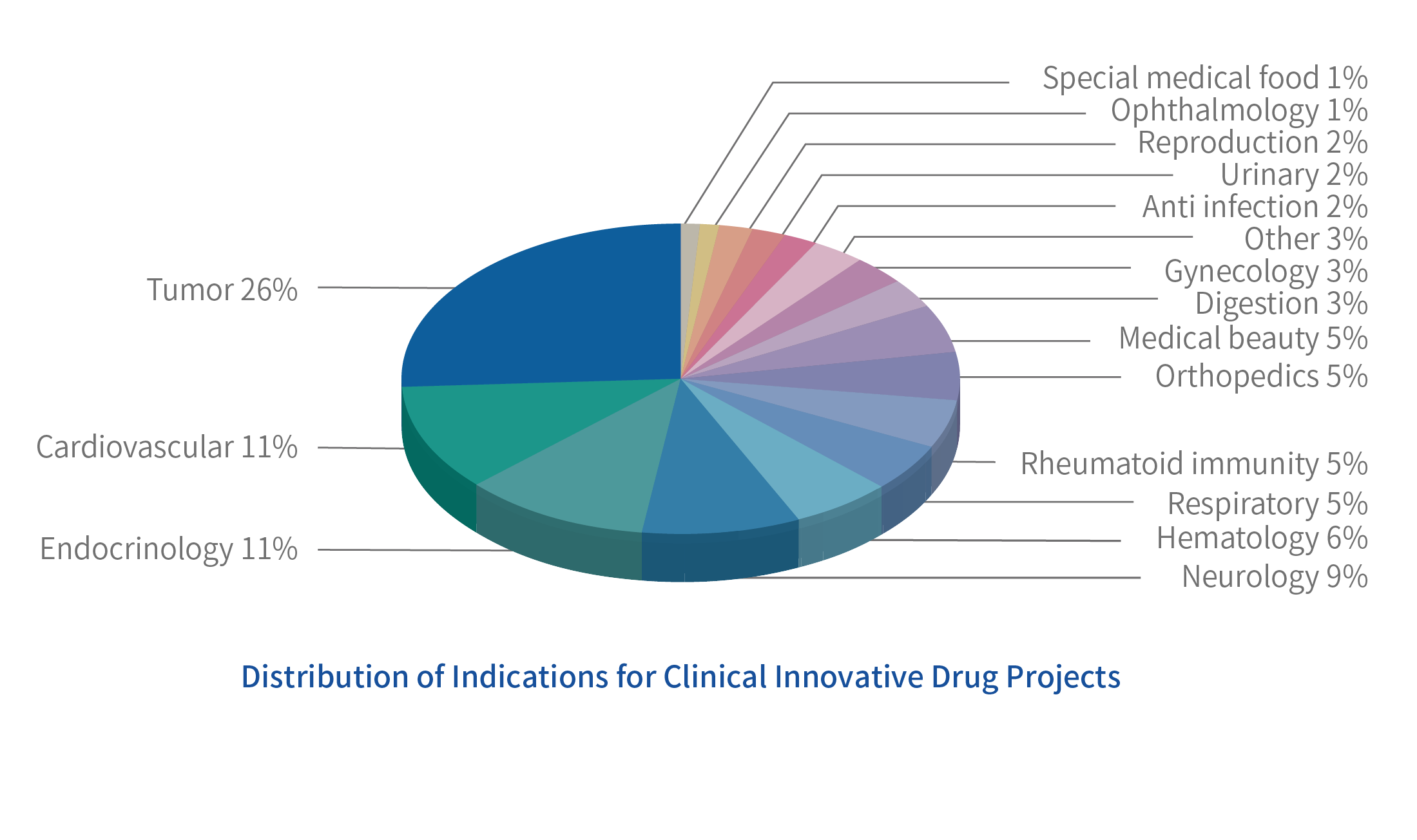
We has provided diversified R&D support clinical technical services for more than 500 well-known enterprises at home and abroad, including clinical trials of innovative/generic drugs of chemical drugs, traditional Chinese medicine, biological drugs, clinical studies of bioequivalence (BE) and pharmacokinetics (PK/PD) of generic drugs, in vivo and in vitro correlation studies (IVIVR), medical scheme design and guidance, third-party audit services, project investment and financing, etc., covering large and small molecule drugs Meet the customized needs of clinical research of different R&D enterprises, and open up the key bottleneck before new drugs are launched for domestic and foreign customers.
Among them, the clinical medical team of Leadingpharm has more than 50 people with professional medical background, focusing on medical services. Rich experience in the design of generic and innovative drug schemes and clinical development strategies, internal and external expert resources in all dimensions, and clinical trial cooperation with a number of well-known domestic and foreign pharmaceutical enterprises and top three hospitals to promote product application and listing with high efficiency and quality based on clinical needs. Assist the enterprise to implement the product clinical research and development strategy, IND data writing, medical research, clinical trial program design, ethical data writing, medical training and other related medical services.



 Hot line:010-61006450
Hot line:010-61006450
 EN
EN
 010-61006450
010-61006450 Address:
Address: Marketing Department:
Marketing Department: Leadingpharm
Leadingpharm Pharm News
Pharm News 010-61006450
010-61006450
